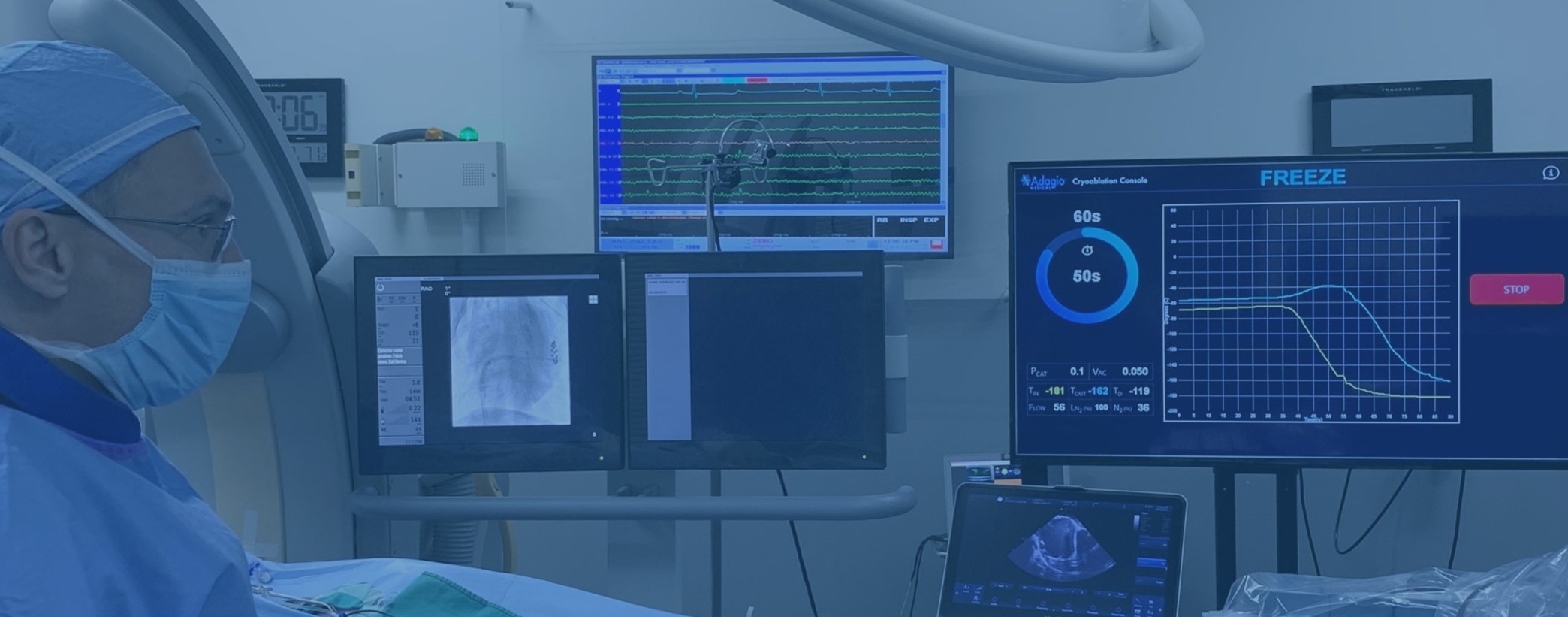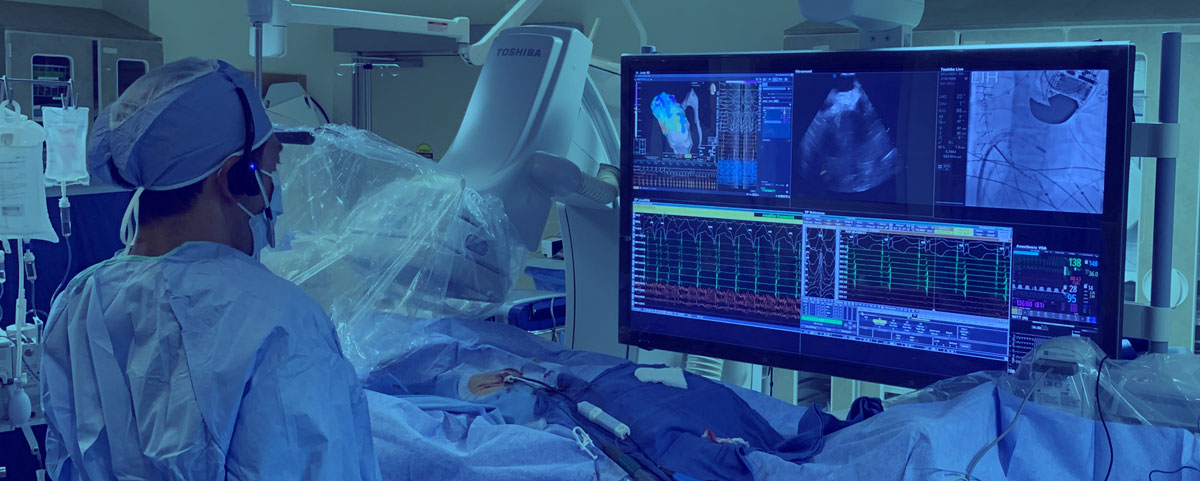
vCLAS™ Purpose-Build CatheterA New Benchmark in VT Ablations
TECHNICAL DETAIL AND CLINICAL RESEARCH
VT ultra-low temperature cryoablation (ULTC) system utilizes -196˚C nitrogen flow to rapidly freeze myocardial tissue to form extensive and durable endocardial lesions 1, required for successful ablation of scar-mediated monomorphic ventricular tachycardia in patients with ischemic and non-ischemic cardiomyopathy. The system consists of the Cryoablation Console and vCLAS™ catheter designed specifically to address the challenges of VT ablations. The data from the multi-center European trial of the VT Cryoablation System showed 97.1% acute elimination of clinical VTs in procedures averaging 188 minutes in duration with the average of 8.9 ± 4.3 lesions and 0% major adverse events. At 6-months follow-up, the freedom from ICD shock was 81.0%, with no significant difference between patients with ischemic and non-ischemic cardiomyopathy, and with significant and sustained reduction in both VT burden and use of amiodarone2.
FULCRUM-VT (Feasibility of Ultra-Low Temperature Cryoablation in Recurring Monomorphic Ventricular Tachycardia, NCT05675865) is a prospective, multi-center, open-label, single-arm study, enrolling 206 patients with structural heart disease of both ischemic and non-ischemic cardiomyopathy, indicated for catheter ablation of drug refractory ventricular tachycardia in accordance with current treatment guidelines. The results of the study will be used to obtain premarket approval from the FDA for Adagio's VT Cryoablation System with the vCLAS cryoablation catheter with industry's broadest indication for purely endocardial ablation of scar-mediated VT. FULCRUM-VT is active enrolling patients.
The bi-directionally deflectable vCLAS catheter features a 15-millimeter long ULTC ablation element, capable of creating lesions with titratable width and depth exceeding 10 millimeters, making it time- and effort-efficient across the range of purely endocardial ablation strategies in patients with both ischemic and non-ischemic cardiomyopathies,2 including those with otherwise challenging mid-myocardial scarring.3The use of cryogenic energy and associated cryoadhesion ensures catheter stability during ablation while the absence of catheter irrigation simplifies the hemodynamic management of patients who often present with symptoms of clinical heart failure.
FULCRUM-VT (Feasibility of Ultra-Low Temperature Cryoablation in Recurring Monomorphic Ventricular Tachycardia, NCT05675865) is a prospective, multi-center, open-label, single-arm study, enrolling 206 patients with structural heart disease of both ischemic and non-ischemic cardiomyopathy, indicated for catheter ablation of drug refractory ventricular tachycardia in accordance with current treatment guidelines. The results of the study will be used to obtain premarket approval from the FDA for Adagio's VT Cryoablation System with the vCLAS cryoablation catheter with industry's broadest indication for purely endocardial ablation of scar-mediated VT. FULCRUM-VT is active enrolling patients.
The bi-directionally deflectable vCLAS catheter features a 15-millimeter long ULTC ablation element, capable of creating lesions with titratable width and depth exceeding 10 millimeters, making it time- and effort-efficient across the range of purely endocardial ablation strategies in patients with both ischemic and non-ischemic cardiomyopathies,2 including those with otherwise challenging mid-myocardial scarring.3The use of cryogenic energy and associated cryoadhesion ensures catheter stability during ablation while the absence of catheter irrigation simplifies the hemodynamic management of patients who often present with symptoms of clinical heart failure.
The Adagio Medical Inc. VT Cryoablation System (vCLAS Catheter and Cryoablation Console) is an investigational device, limited by Federal law to investigational use.
1 F. Bourier et al, J Cardiovasc Electrophysiol. 2021 Mar; 32(3):570-577
2 A. Verma et al., Cryocure-VT: the safety and effectiveness of ultra-low-temperature cryoablation of monomorphic ventricular tachycardia in patients with ischaemic and non-ischaemic cardiomyopathies. Europace 2024;26:euae076
3 Dilk P, Darma A, Hindricks G, Dinov B. Ultra-low-temperature cryoablation for ventricular tachycardia in nonischemic cardiomyopathy – a case report. Heart Rhythm Case Reports 2023;9:469-472
2 A. Verma et al., Cryocure-VT: the safety and effectiveness of ultra-low-temperature cryoablation of monomorphic ventricular tachycardia in patients with ischaemic and non-ischaemic cardiomyopathies. Europace 2024;26:euae076
3 Dilk P, Darma A, Hindricks G, Dinov B. Ultra-low-temperature cryoablation for ventricular tachycardia in nonischemic cardiomyopathy – a case report. Heart Rhythm Case Reports 2023;9:469-472