
For Patients and CaregiversIf You Were Diagnosed...
CATHETER ABLATION IN TREATMENT OF CARDIAC ARRHYTHMIAS
Cardiac arrhythmias (both atrial and ventricular) represent significant healthcare burden worldwide and negatively affect patient's quality of life. Minimally-invasive catheter ablation therapies have been proven effective in treating cardiac arrhythmias and are becoming the first-choice treatments for certain patients and conditions. You can learn more about cardiac arrhythmias and treatment choices by following these links:
• American Heart Association - role of ablation in treatment of arrhythmias
• European Heart Rhythm Association - general information about catheter ablation for atrial fibrillation
• WebMD - general information about catheter and surgical ablations for atrial fibrillation
• American Heart Association - role of ablation in treatment of arrhythmias
• European Heart Rhythm Association - general information about catheter ablation for atrial fibrillation
• WebMD - general information about catheter and surgical ablations for atrial fibrillation
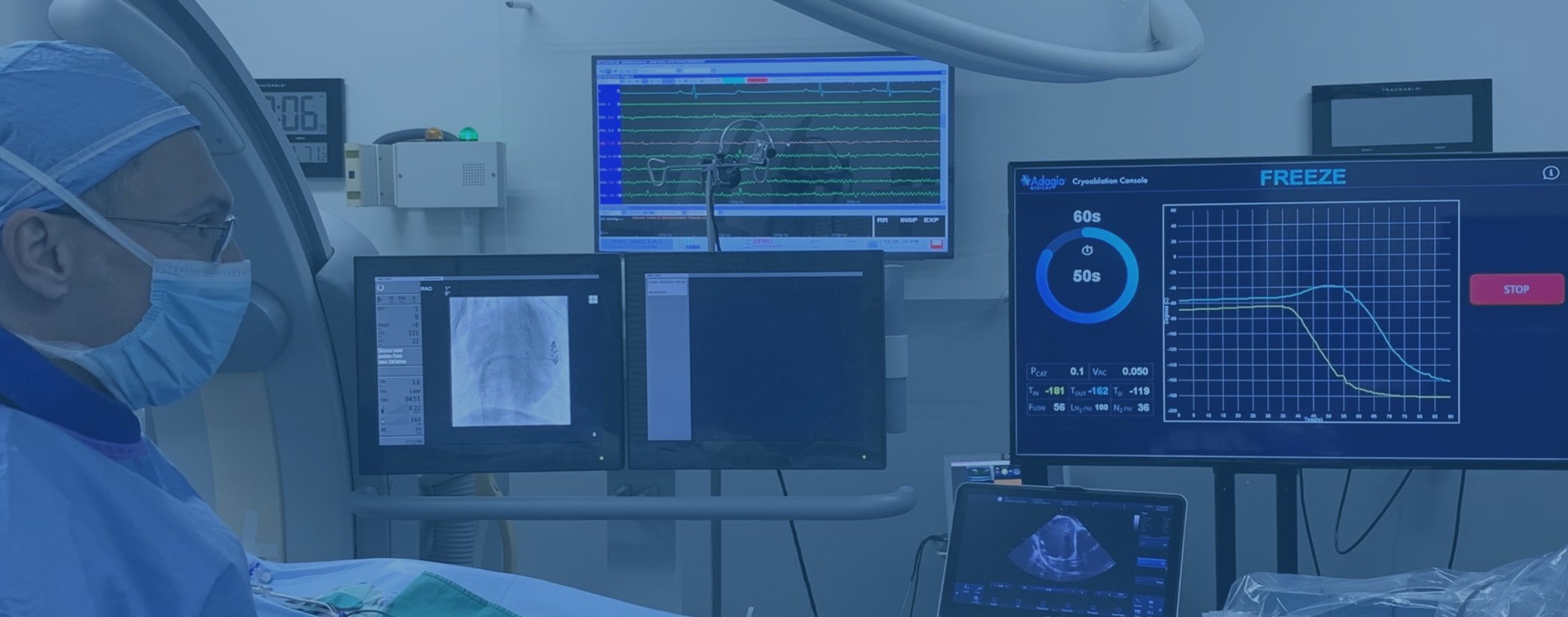 DISCOVER ADAGIO MEDICAL TECHNOLOGY
DISCOVER ADAGIO MEDICAL TECHNOLOGY see more >
During catheter ablation, physicians use electric or thermal energy to modify the conducting properties of your heart in order to interrupt the abnormal electric circuits that lead to arrhythmia. The key to the effective ablation is to ensure that these electric circuits are fully disconnected and do not reconnect. The Adagio Medical has developed a set of unique minimally-invasive technologies that aim to accomplish this goal with three innovative features:
• The use of liquid nitrogen as a source of cryogenic energy to ensure deep and permanent effect on heart tissue. This technology is known as Ultra-Low Temperature Cryoablation or ULTC.
• vCLAS' ULTC catheter, purpose-built for ablation of ventricular tachycardia, which has unique functional benefits in these otherwise complex and potentially long procedures.
• The integration (where applicable) of cryogenic energy with pulsed electric field energy to further improve procedure effectiveness and long-term patient outcomes. This technology, currently in early evaluation, is called Pulsed Field Cryoablation or PFCA.
• The use of liquid nitrogen as a source of cryogenic energy to ensure deep and permanent effect on heart tissue. This technology is known as Ultra-Low Temperature Cryoablation or ULTC.
• vCLAS' ULTC catheter, purpose-built for ablation of ventricular tachycardia, which has unique functional benefits in these otherwise complex and potentially long procedures.
• The integration (where applicable) of cryogenic energy with pulsed electric field energy to further improve procedure effectiveness and long-term patient outcomes. This technology, currently in early evaluation, is called Pulsed Field Cryoablation or PFCA.
 EXPLORE CLINICAL TRIAL PARTICIPATION
EXPLORE CLINICAL TRIAL PARTICIPATION see more >
Adagio Medical is currently conducting the FULCRUM-VT pivotal IDE trial for VT Cryoablation System with vCLAS' catheter for treatment of monomorphic ventricular tachycardia. The system has CE-mark approval and is in active clinical use in Europe; this clinical trial is being conducted to fulfill U.S. FDA requirements for product approval*.
If you were diagnosed with monomorphic ventricular tachycardia and your physician has discussed the risks and benefits of catheter ablations in addition to antiarrhythmic medication to control your symptoms, you may be eligible for trial participation. By participating, you will receive the most advanced procedure using vCLAS™ catheter and closer clinical follow-up, while helping to bring this innovative technology to patients in the U.S.
*The FULCRUM-VT clinical study evaluates the safety and effectiveness of the Adagio VT Cryoablation System in the ablation treatment of sustained monomorphic ventricular tachycardia. Data will be used to support an application for premarket approval (PMA).
If you were diagnosed with monomorphic ventricular tachycardia and your physician has discussed the risks and benefits of catheter ablations in addition to antiarrhythmic medication to control your symptoms, you may be eligible for trial participation. By participating, you will receive the most advanced procedure using vCLAS™ catheter and closer clinical follow-up, while helping to bring this innovative technology to patients in the U.S.
*The FULCRUM-VT clinical study evaluates the safety and effectiveness of the Adagio VT Cryoablation System in the ablation treatment of sustained monomorphic ventricular tachycardia. Data will be used to support an application for premarket approval (PMA).